
WELCOME TO i4KIDS-4RARE:

THE PROJECT

The i4KIDS-4RARE project is designed to address the critical medical, research and social needs associated with orphan medical devices for rare diseases:
Overall, i4KIDS-4RARE not only addresses urgent medical needs, but also creates a positive social impact and supports ongoing research that is changing the landscape of rare disease management.
Medical needs
Research needs
Social needs
i4KIDS-4RARE Accelerator Programme
• Challenge-based Clinical 4RARE Programme (with clinical and industry focus):
Use Case #1: paediatric epilepsy (neurology)
• Validation & Valorisation 4RARE programme:
Use Case #2: Congenital Heart Disease (Cardiology)
Use Case #3: Spinal Muscular Atrophy (SMA) (Rehabilitation)
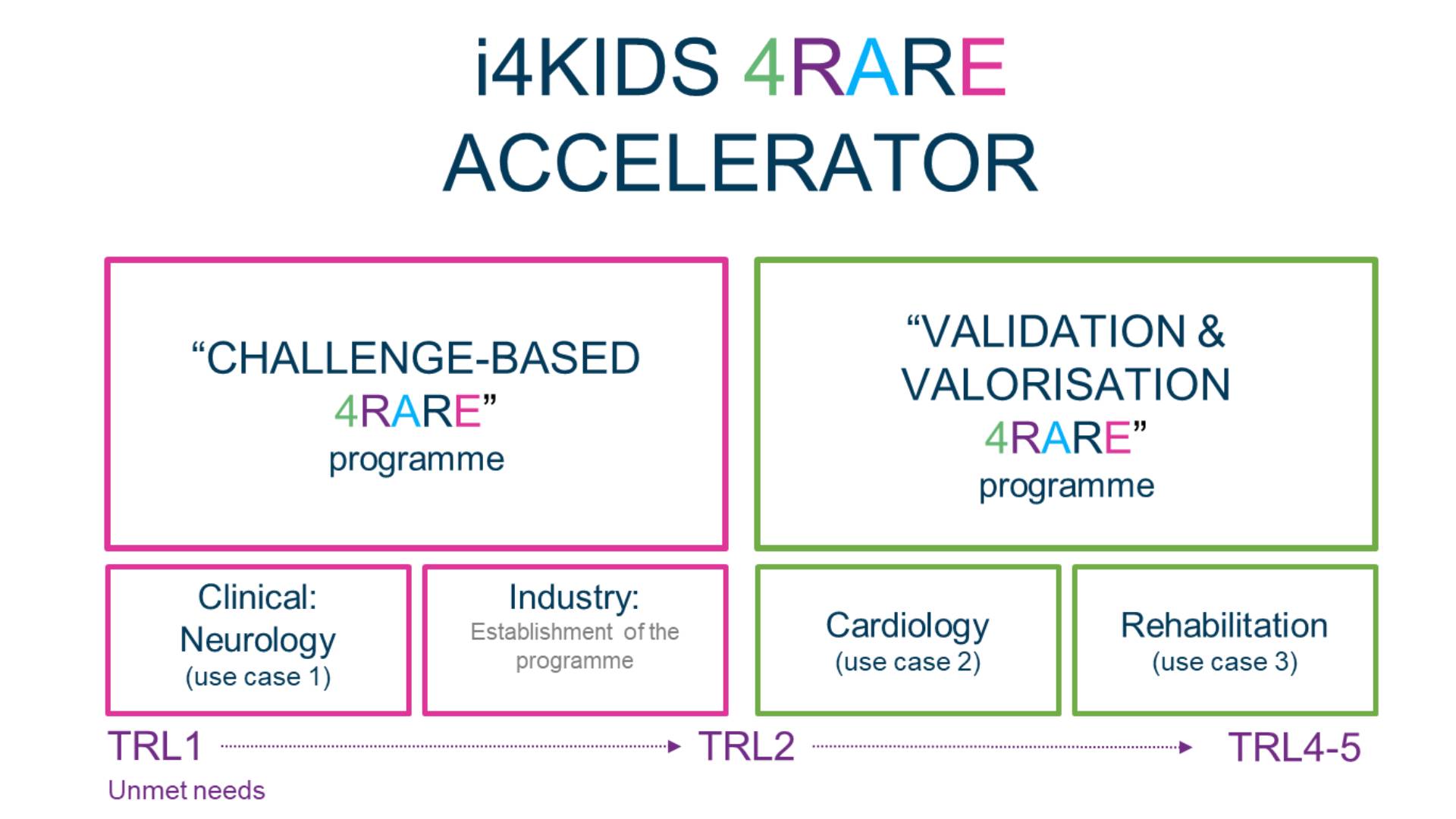
In detail, the three use cases to be supported by i4KIDS-4RARE are as follows:
Use case #1 – Paediatric Epilepsy (Neurology)


Use case #2 – Congenital Heart Disease (Cardiology)
Use case #3 – Spinal Muscular Atrophy (SMA) (Rehabilitation)
Rehabilitation for non-ambulatory SMA patients can be repetitive and difficult, leading to challenges in maintaining motivation and adherence. Travel to rehabilitation centres can also be burdensome. To address these issues, the Sant Joan de Déu Barcelona Children’s Hospital rehabilitation and innovation teams developed a virtual reality (VR) video game to support home rehabilitation for SMA-II and III patients. This innovative approach allows for monitored rehabilitation exercises, tracking progress, and enhancing treatment adherence. The project has been selected to enter the validation phase of the Acceleration-4RARE programme, focusing on technical proof of concept and market access strategies. If successful, the i4KIDS-4RARE team will work on the regulatory roadmap to move towards broader implementation.


Beneficiary and coordinator

Affiliated Entity
